
Long-lasting relief from bladder and bowel control issues
It's time to find real relief.
See if Axonics® Therapy can help you restore normal bladder and bowel activity and find long-lasting relief.

Long-lasting relief from bladder and bowel control issues
See if Axonics® Therapy can help you restore normal bladder and bowel activity and find long-lasting relief.
Axonics Therapy is an advanced treatment option that can help restore normal control of the bladder and bowel and may provide life-changing results when other treatment options have failed.
Axonics Therapy is indicated for treating symptoms of:
 Overactive Bladder
Overactive Bladder
 Bowel Incontinence
Bowel Incontinence
 Urinary Retention
Urinary Retention
Axonics Sacral Neuromodulation Therapy provides gentle stimulation to the nerves that control the bladder and bowel. The stimulation can restore normal communication between the brain and the bladder and bowel.
Sacral Neuromodulation has been an available treatment for bladder and bowel control issues for years but is continually advancing. Axonics Therapy uses the latest technology and is clinically proven to help people regain control of their normal life when other treatments have failed.
Hear from an expert about who might be a good candidate for Axonics Therapy
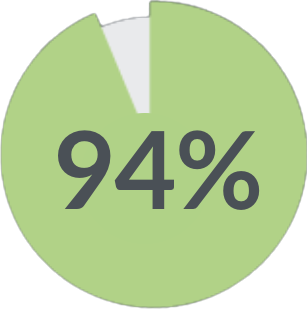
of patients were satisfied with their therapy 1
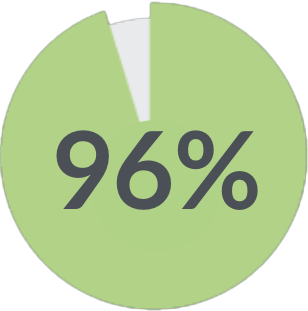
of patients say the device is easy to use 1
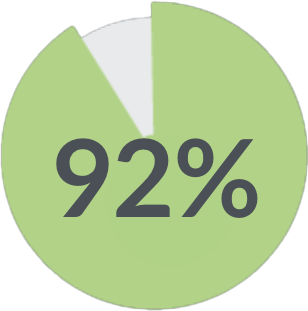
of patients said they would undergo treatment again with the same expected results 1

“The procedure was so easy with no pain or discomfort. I wish I would have done it 10 years ago because now I have my life back.”
- Donna H.

"This therapy makes me feel young again because now I can do anything."
- Kathy A.
*Results and experiences may vary and are unique to each patient. No promise or guarantee is made about specific results and experiences. Please click here for important safety information.
Find out if Axonics Therapy can help you restore normal bladder and bowel activity and find long-lasting relief.
1. Benson K, McCrery R, Taylor C, et al. One-year outcomes of the ARTISAN-SNM study with the Axonics System for the treatment of urinary urgency incontinence. Neurourology and Urodynamics. 2020;1-7. https://doi.org/10.1002/nau.24376
IMPORTANT SAFETY INFORMATION:
Indications:
Axonics SNM Therapy for urinary control is indicated for the treatment of urinary retention and the symptoms of overactive bladder, including urinary urge incontinence (leakage) and significant symptoms of urgency-frequency, either alone or in combination. Axonics SNM Therapy for bowel control is indicated for the treatment of chronic fecal incontinence. The therapy should only be used in patients who have failed or are not candidates or could not tolerate more conservative treatments.
Contraindications:
Axonics SNM Therapy is contraindicated for patients who have not demonstrated an appropriate response to test stimulation or patients who are unable to operate Axonics SNM Therapy.
Warnings:
Implantation and use of Axonics Therapy incurs risk beyond those normally associated with surgery, some of which may necessitate surgical intervention. These risks include, but are not limited to adverse change in voiding function (bowel and/or bladder), infection, pain or irritation at the implant site, lead or device migration, electrical shock, change in sensation or magnitude of stimulation which has been described as uncomfortable (jolting or shocking) by some patients, and heating or burns at the device site. Results and experiences may vary and are unique to each patient. No promise or guarantee is made about specific results or experiences. Talk to your doctor about whether Axonics Therapy is right for you and to discuss the potential risks and benefits.
For more information about safety and potential risks, go to www.axonics.com/isi.
Precautions:
The safety and effectiveness of Axonics Therapy has not been established for use in women who are pregnant or in delivery; for pediatric patients (under the age of 18 years for fecal incontinence and under the age of 16 years for overactive bladder and urinary retention); for patients with neurological diseases, such as multiple sclerosis or diabetes, or for bilateral stimulation.
Caution: U.S. federal law restricts this device to sale and use by, or on the order of, a physician.